Dong-Hee Lim
Chungbuk National University, South Korea
Title: First principles understanding on enhanced oxidation resistance of NaBH4-treated mackinawite (FeS)
Biography
Biography: Dong-Hee Lim
Abstract
Sulfide minerals are important in immobilizing toxic pollutants in reducing environments. Iron sulfide (FeS) is ubiquitous in anoxic conditions and is a good remover of various organic contaminants and heavy metals. This study was conducted to modify the FeS synthesis process to delay the air oxidation rate of FeS by adding NaBH4. Also, the fundamental mechanism of how NaBH4 enhanced the oxidation resistance of FeS was theoretically investigated by the density functional theory (DFT) calculations. The real-time oxidation test results showed that the suggested NaBH4-modification retarded the oxidation of FeS by 8 times compared to the unmodified FeS. The DFT results revealed that the FeS oxidation was attributed to sulfide oxidation of S-terminated FeS due to strong electronegativity of oxygen, and by treating FeS with the hydride ion (H−) donated from the ionization of NaBH4, less charge was withdrawn from the FeS sulfur atoms (i.e., retarded FeS oxidation) because oxidizing environment preferentially withdrew charge from the hydride ion (H−) rather than from FeS. The proposed FeS modification process may be useful in simplifying the application of reduced iron- or sulfide-minerals as alternatives to ZVI or oxide-based metal absorbents in environmental technologies. The real-time XAS measurements demonstrated that it is a useful method for accelerating kinetically slow or quasistatic equilibrium reactions. The pollutant removal efficiency of the modified FeS, compared to the unmodified FeS, will be evaluated in future studies.
Image
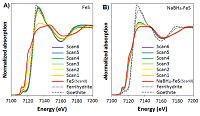
Figure 1: Fe K-edge XANES of (A) FeS and (B) NaBH4-FeS. Consecutive scans taken under atmospheric conditions were numbered from Scan 1 to 6. Scans 0 represent the XANES scans of unoxidized FeS and NaBH4-FeS. All model compounds (unoxidized FeS and NaBH4-FeS, ferrihydrite, and goethite) were scanned under a He flow, to prevent exposure to air during the measurements.