Young-Soo Han
Korea Institute of Geoscience and Mineral Resources, South Korea
Title: Interaction of Sb(III) under sulfide-rich reducing environment: Batch and spectroscopic investigations
Biography
Biography: Young-Soo Han
Abstract
This study examined the reaction mechanism of antimonate Sb(III) uptake in iron monosulfide mackinawite and compared its removal capacity and pH-dependent uptake behavior with that of As(III). The comparison of Sb(III) with As(III), based on their chemical similarity, may give useful insight into the chemical properties of the less studied Sb(III). Batch sorption studies revealed that Sb(III) had a higher affinity for mackinawite at pH 5 than pH 7 or 9. While As(III) displayed a similar trend, there was a much higher uptake of Sb(III) under all three pH conditions. A spectroscopic study demonstrated the high uptake of Sb(III) at pH 5 was due to precipitation of the sulfide mineral Sb2S3 as a consequence of the mackinawite dissolution while the removal at pH 7 or 9 was inferred as a surface reaction possibly a sole or mixed reactions of adsorption and surface-precipitation. These pH-dependent Sb(III) uptake mechanisms are similar to the corresponding mechanisms for As(III) uptake, demonstrating that mackinawite is also a good scavenger for Sb(III) in ferrous and sulfide-rich reducing environment like for As(III).
Image
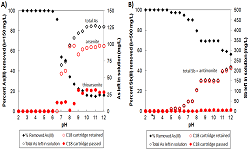
Figure 1: As(III) and Sb(III) removal efficiency under pH 2 to 12 with the amount of dissolved As or Sb left in solution with its speciation (I0 = 150 mg/L for As(III) and 500 mg/L for Sb(III)). Aqueous speciation was conducted using Bond Elut C18 cartridge
Speaker Presentations
Speaker PDFs
Speaker PPTs Click Here